
Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA). The Vaccine Adverse Event Reporting System ( VAERS) is a United States program for vaccine safety, co-managed by the U.S.
#Vaers database plus
COVID-19 Vaccines are Effective plus icon.Investigating Long-Term Effects of Myocarditis.Possibility of COVID-19 Illness after Vaccination.

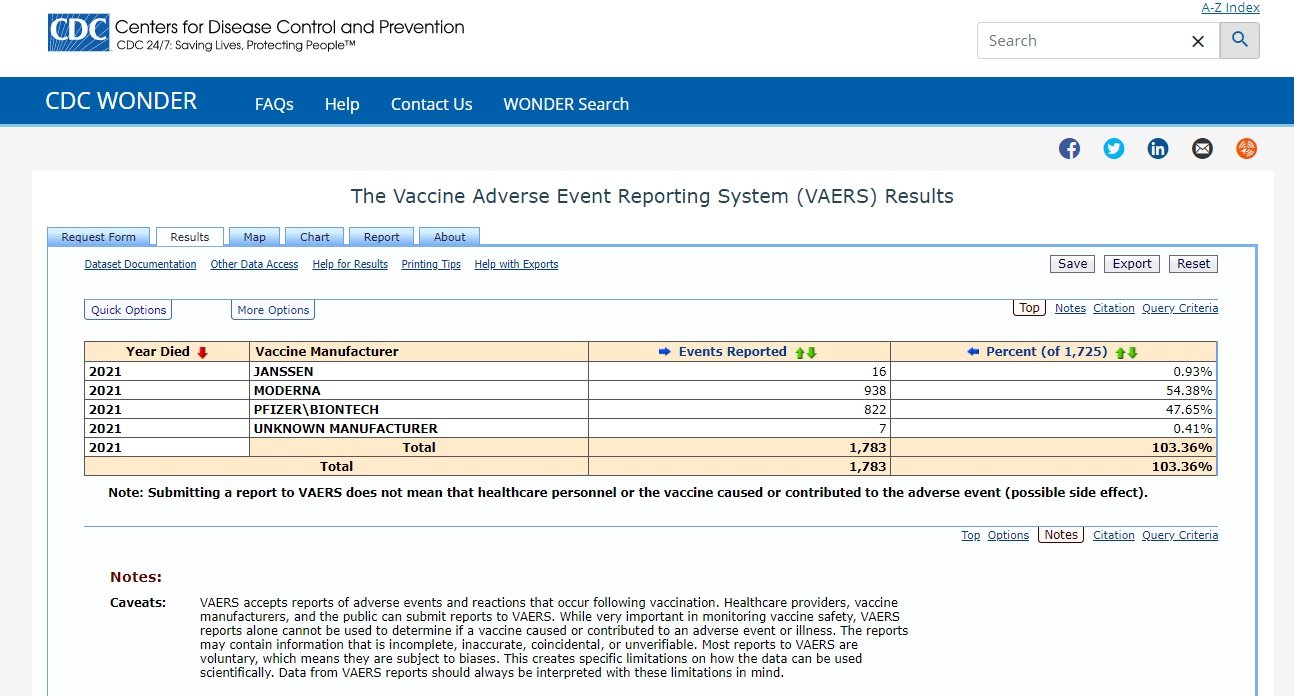
Stay Up to Date with Vaccines plus icon.Why Children & Teens Should Get Vaccinated.CDC and FDA clinicians review reports of death to VAERS including death certificates, autopsy, and medical records. During this time, VAERS received 15,700 preliminary reports of death (0.0026%) among people who received a COVID-19 vaccine. More than 603 million doses of COVID-19 vaccines were administered in the United States from December 14, 2020, through July 27, 2022.

Reports of adverse events to VAERS following vaccination, including deaths, do not necessarily mean that a vaccine caused a health problem. FDA requires healthcare providers to report any death after COVID-19 vaccination to VAERS, even if it’s unclear whether the vaccine was the cause. Reports of death after COVID-19 vaccination are rare.Learn more about myocarditis and pericarditis, including clinical considerations, after mRNA COVID-19 vaccination. See below for counts of verified reports of myocarditis by age group.ĥ-11 years: 22 verified reports of myocarditis after 20,404,074 doses administeredġ2-15 years: 346 verified reports of myocarditis after 24,198,309 doses administeredġ6-17 years: 297 verified reports of myocarditis after 13,326,016 doses administeredĪs the COVID-19 vaccines are authorized for younger children, CDC and FDA will continue to monitor for and evaluate reports of myocarditis and pericarditis after COVID-19 vaccination and will share more information as it becomes available. Through confirmation of symptoms and diagnostics by provider interview or review of medical records, 665 reports have been verified to meet CDC’s working case definition for myocarditis. Most cases have been reported after receiving Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines), particularly in male adolescents and young adults.Īs of July 28, 2022, there have been 1,010 preliminary reports in VAERS among people younger than age 18 years under review for potential cases of myocarditis and pericarditis. Most patients with myocarditis or pericarditis after COVID-19 vaccination responded well to medicine and rest and felt better quickly. Myocarditis is inflammation of the heart muscle, and pericarditis is inflammation of the outer lining of the heart. Myocarditis and pericarditis after COVID-19 vaccination are rare.CDC and FDA will continue to monitor for and evaluate reports of GBS occurring after COVID-19 vaccination and will share more information as it becomes available. The analysis found no increased risk of GBS after Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines). After the first 42 days, the rate of GBS was 11 times higher following J&J/Janssen COVID-19 vaccination. Based on a recent analysis of data from the Vaccine Safety Datalink, the rate of GBS within the first 21 days following J&J/Janssen COVID-19 vaccination was found to be 21 times higher than after Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines).


 0 kommentar(er)
0 kommentar(er)
